Quantum Numbers: H to Ne
There are four quantum numbers: n, ℓ, mℓ, and ms. Each one is a particular factor in an equation describing a property of the electron. At this introductory level, the equations are not needed. The value of each quantum number is assigned to each electron in an atom by a 'building up' process. Niels Bohr called this process the 'Aufbau' principle: aufbau means 'building up.'
n is ALWAYS the starting point for building up a series of quantum numbers. Each quantum number is then assigned according to a set of rules, each of which took years of study to finally determine. The rules ARE NOT just any old arbitrary ones; they have been determined from a study of nature. Remember the rules:
(1) n = 1, 2, 3, and so on.
(2) ℓ = 0, 1, 2, . . . , n - 1
(3) mℓ starts at negative ℓ, runs by whole numbers to zero and then goes to positive ℓ.
(4) after the n, ℓ and mℓ to be used have been determined, assign the ms value +½ to one electron, then assign the ms value of -½ to the next electron, while using the same n, ℓ and m values.
Orbital diagram, after Barrett (2002), showing the participating atomic orbitals from each oxygen atom, the molecular orbitals that result from their overlap, and the aufbau filling of the orbitals with the 12 electrons, 6 from each O atom, beginning from the lowest energy orbitals, and resulting in covalent double bond character from filled orbitals (and cancellation of the contributions of the pairs of σ and σ. and π and π. orbital pairs). In case of OXYGEN, you already know that the atomic number tells you the number of electrons. That means there are 8 electrons in an oxygen atom. Lacetti service manual. Of Oxygen=8 electrons 8= (2,6).
Also, keep in mind that we use only one n, ℓ, mℓ, and ms value each to make a set of four quantum numbers for each electron. It is this set of four quantum numbers that uniquely identifies each electron.
Last point: the last column in each table below is called 'Orbital Name.' As you are reading this tutorial, you may not yet know what an orbital is. That's OK, but please understand the concept called 'orbital' is an important one. Here's a real simple description that ignores lots of details: each orbital is a region of space around the nucleus which contains a MAXIMUM of two electrons. Realize that it's more complex than that, but the above description is good enough for now. I hope!!
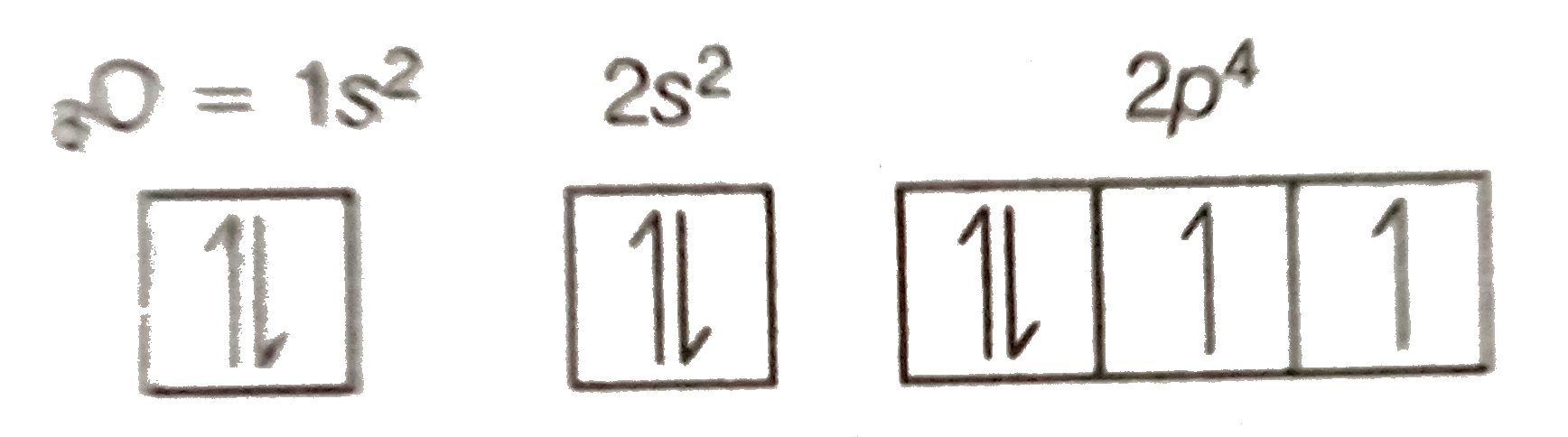
Hydrogen - one electron
First Electron
n = 1
ℓ = 0
mℓ = 0
In each case, note that we start with the smallest value of n, ℓ, or mℓ possible. Make sure you look over the rules to see how each value was arrived at. ℓ starts at zero and goes to n - 1, which is zero since we get 1 - 1 = 0, when using n = 1. When ℓ = 0, there is only one possible choice for mℓ Gmail account creator. , which must be zero.
ms = +½
This completes the four quantum numbers for the single electron possessed by hydrogen. I shall build up a table like this:

| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 1 | Hydrogen | 1 | 0 | 0 | +½ | 1s |
Helium - two electrons
First Electron
n = 1
ℓ = 0
mℓ = 0
ms = +½
The first electron in helium has exactly the same four quantum number of the first electron in hydrogen. However, helium has TWO electrons. So we 'build up' from the previous electrons by adding one more.
Second Electron
n = 1
ℓ = 0
mℓ = 0
ms = -½
Notice the same n, ℓ, and mℓ values, but ms has shifted from positive ½ to negative ½. This was the problem Pauli saw in 1925. Three quantum numbers was insufficient to UNIQUELY identify each electron, but a fourth one (the one called ms) did the trick.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 2 | Helium | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ |
Lithium - three electrons
The first two electrons quantum numbers' are EXACTLY the same as the two in helium:
1, 0, 0, +½ and 1, 0, 0, -½
Third Electron: here's where we 'build up' by adding one more electron.
However, we are now presented with a problem. All the values with n = 1 have been used up, but we have only accounted for two of lithium's three electrons. What to do about the third?
Answer: start with the NEXT n value; n = 2. However, there is a problem with ℓ; do we use ℓ = 0 or ℓ = 1, since both are possible with n = 2?
Answer: start with the lowest value first, so that means using ℓ = 0. (Don't worry, we will use ℓ = 1 soon enough.)
Figuring out mℓ should be easy; when ℓ = 0, mℓ can only equal 0. So n, ℓ, mℓ for the third electron is 2, 0, 0. I'll add in ms in the table below.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 3 | Lithium | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s |

Beryllium - four electrons
In the building up process, we go one electron at a time. Therefore, we will use the three from lithium and add one more.
Fourth Electron
n = 2
ℓ = 0
mℓ = 0
ms = -½
Notice the same n, ℓ, and m values as the third electron, but ms for the fourth electron has shifted from positive ½ to negative ½.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 4 | Beryllium | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ |
Pretty easy, eh? It stays easy, if you follow the rules. With beryllium, we have exhausted the possibilities for the n = 2; ℓ = 0 combination. However, when n = 2, ℓ can take on another value, namely ℓ = 1. This has consequences for the mℓ value as well and, after we finish, there will be six electrons that have a combination of n = 2 and ℓ = 1.
Here's the rule for mℓ again: start at negative ℓ, run by whole numbers to zero and then go to positive ℓ. Since ℓ = 1, we start with -1, go to zero and end up at +1. This gives us three values for mℓ when ℓ = 1. Hopefully you can see that, since ms takes on +½ and -½, we will wind up with six sets of quantum numbers.
Warning: there's going to be a new rule introduced after boron. So prepare yourself because, just as you thought it was getting easy, there gets added some new stuff. By the way, us mean old teachers didn't make all this stuff up to torture poor chemistry students. Nature really does do what I will explain below. Here's boron:
Boron - five electrons
Following the usual pattern, I've repeated the previous four electrons. As we go on to the ℓ = 1 values, keep in mind that we will start with the lowest value of mℓ, namely negative one.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 5 | Boron | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ | |||
| 2 | 1 | -1 | +½ | 2px |
Eventually, I will wind up with three orbital names. 2px is just the first, x meaning the x-axis. Next will be 2py, for the y-axis and the last name used will be 2pz, for the z-axis. These three orbitals are oriented at 90° to each other.
Hund's Rule (named for Fredrich Hund) is the name of the new rule. This rule concerns the relationship between the ℓ and mℓ quantum numbers. When ℓ = 0, mℓ can only equal zero and Hund's Rule does not show up. However, now that we have reached ℓ = 1, mℓ can take on multiple values. Hund's Rule concerns the order in which we assign the ℓ and mℓ values.
By the way, I'm going to avoid a technical statement of Hund's Rule for the moment. I'll discuss how it works first.
Hund's Rule means that we will use each possible ℓ, mℓ combination ONCE before going back and using it a second time. Here are the three possible ℓ, mℓ combos when ℓ = 1: Aramco salary scale pdf files.
| ℓ | mℓ |
| 1 | -1 |
| 1 | 0 |
| 1 | +1 |
For boron, we have used the ℓ, mℓ combination of 1, -1. The key is to see that Hund's Rule requires we go on to the NEXT ℓ, mℓ combination for the next element: carbon.
Carbon - six electrons
Following the usual pattern, I've repeated the previous five electrons. As we continue on with the ℓ = 1 values, keep in mind that Hund's Rule will affect how we assign the next mℓ value.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 6 | Carbon | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ | |||
| 2 | 1 | -1 | +½ | 2px | ||
| 2 | 1 | 0 | +½ | 2py |
Nitrogen - seven electrons
Since we still have not first used all possible ℓ, mℓ values ONCE, we go on to the next ℓ, mℓ combination.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 7 | Nitrogen | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ | |||
| 2 | 1 | -1 | +½ | 2px | ||
| 2 | 1 | 0 | +½ | 2py | ||
| 2 | 1 | +1 | +½ | 2pz |
2px, 2py and 2pz are three different orbitals, each one capable of holding two electrons. Notice how, in nitrogen, each of the three orbitals is filled up HALF-WAY (that is, with one electron) before we go back and fill up each orbital with the second electron.
This 'half-filled orbital' has definite chemical consequences. Remember it well. Also, using 2px first, then going to y and then z is purely convention. The x, y, z order is not of consequence in the above examples. However keep in mind the using each letter ONCE first being using it for the second electron is important.

Oxygen - eight electrons
Electrons In Oxygen Outer Shell
Now that we have used each ℓ, mℓ combination once, we proceed to go back and use each combo the second time. For oxygen to neon, I've marked which electron is the one added.
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 8 | Oxygen | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ | |||
| 2 | 1 | -1 | +½ | 2px | ||
| this one added | ---> | 2 | 1 | -1 | -½ | |
| 2 | 1 | 0 | +½ | 2py | ||
| 2 | 1 | +1 | +½ | 2pz |

Fluorine - nine electrons
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 9 | Fluorine | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ | |||
| 2 | 1 | -1 | +½ | 2px | ||
| 2 | 1 | -1 | -½ | |||
| 2 | 1 | 0 | +½ | 2py | ||
| this one added | ---> | 2 | 1 | 0 | -½ | |
| 2 | 1 | +1 | +½ | 2pz |
Neon - ten electrons
Electrons In Oxygen -2
| Atomic Number | Element | n | ℓ | mℓ | ms | Orbital Name |
| 10 | Neon | 1 | 0 | 0 | +½ | 1s |
| 1 | 0 | 0 | -½ | |||
| 2 | 0 | 0 | +½ | 2s | ||
| 2 | 0 | 0 | -½ | |||
| 2 | 1 | -1 | +½ | 2px | ||
| 2 | 1 | -1 | -½ | |||
| 2 | 1 | 0 | +½ | 2py | ||
| 2 | 1 | 0 | -½ | |||
| 2 | 1 | +1 | +½ | 2pz | ||
| this one added | ---> | 2 | 1 | +1 | -½ |
Electrons In Oxygen 8
We have now completed all possible values for n = 1 AND n = 2. Starting with element 11, sodium, we will proceed on to n = 3. When we finish, we will have used ℓ = 0, ℓ = 1 (and applied Hund's Rule again) and then, before going on to ℓ = 3, we will hit another interesting twist that nature has handed us. We will wind up going on to n = 4 and then coming back to finish n = 3. It will be fun!
